Mass spectrometry is a powerful analytical technique used to quantify known materials, to identify unknown compounds within a sample, and to elucidate the structure and chemical properties of different molecules. The complete process involves the conversion of the sample into gaseous ions, with or without fragmentation, which are then characterized by their mass to charge ratios (m/z) and relative abundances.


Basic Principle
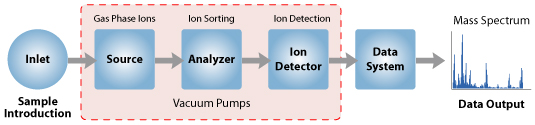
A mass spectrometer generates multiple ions from the sample under investigation, it then separates them according to their specific mass-to-charge ratio (m/z), and then records the relative abundance of each ion type.
Components
The instrument consists of three major components:
- Ion Source: For producing gaseous ions from the substance being studied.
MALDI -TOF: Matrix assisted laser desorption ionization-time of flight
ESI: Electrospray Ionization

- Analyzer: For resolving the ions into their characteristics mass components according to their mass-to-charge ratio.
- Detector System: For detecting the ions and recording the relative abundance of each of the resolved ionic species

- With all the above components, a mass spectrometer should always perform the following processes:
- Produce ions from the sample in the ionization source.
- Separate these ions according to their mass-to-charge ratio in the mass analyzer.
- Eventually, fragment the selected ions and analyze the fragments in a second analyzer.
- Detect the ions emerging from the last analyzer and measure their abundance with the detector that converts the ions into electrical signals.
- Process the signals from the detector that are transmitted to the computer and control the instrument using feedback

No comments:
Post a Comment