This technique was developed by O'Farrel in 1975
Principle: • The method resolves proteins in a protein cocktail in the form of a twodimensional protein map based on their size and charge.
Objective:
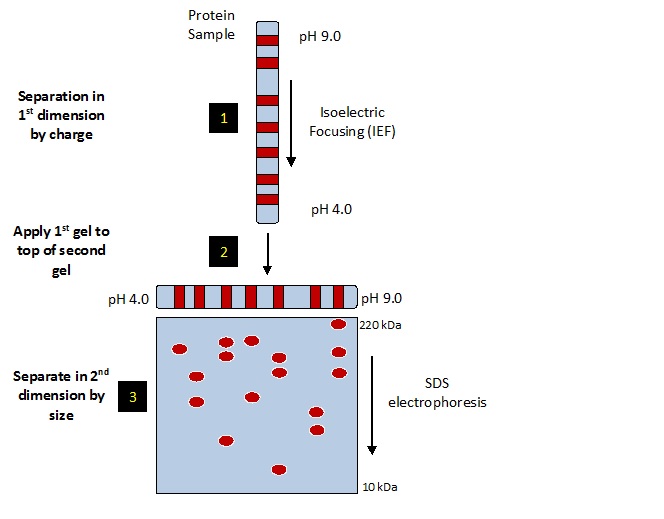
• First dimension: Separation according to proteins isoelectric points (pI)
• Second dimension: Separation according to molecular weight by SDS PAGE
Procedure: •Proteins are separated by their intrinsic charges in a solution which solublizes, denatures and dissociates all the polypeptide charges.
Ist dimension : Isoelectric focusing is used to separate polypeptide chains on the principal of pI.
Isoelectric point (pI) of a protein is the pH at which the protein has a net charge equal to zero. A protein does not move in an electric field when it is at its isoelectric point.
IInd dimension: The polypeptides are separated electrophoretically in polyacrylamide gel which as a pH gradient.
First dimension of the 2D gel electrophoresis is established by the movement of each protein to a position, which corresponds to its isoelectric point in a narrow tube. • This narrow tube is again subjected to electrophoresis in a direction, which is at right angle to the direction that has been used for isolectric focusing. In the second electrophoresis SDS is used to separate the proteins according to their sizes. The second dimension is established by the migration of the separated protein to its discrete spot on the gel.
After fractionation a specific protein can be identified on the gel by radioactive isotope, a detectable enzyme or fluorescent dye.
ISO ELECTRIC FOCUSSING-
Separation of the proteins by isoelectric point is called isoelectric focusing (IEF). When a gradient of pH is applied to a gel and an electric potential is applied across the gel, making one end more positive than the other. • At all pH values other than their isoelectric point, proteins will be charged. If they are positively charged, they will be pulled towards the negative end of the gel and if they are negatively charged they will be pulled to the positive end of the gel. The proteins applied in the first dimension will move along the gel and will accumulate at their isoelectric point; that is, the point at which the overall charge on the protein is 0 (a neutral charge).
SDS - PAGE
In separating the proteins by mass, the gel treated with sodium dodecyl sulfate (SDS) along with other reagents (SDS-PAGE in 1-D). This denatures the proteins (that is, it unfolds them into long, straight molecules) and binds a number of SDS molecules roughly proportional to the protein's length. Because a protein's length (when unfolded) is roughly proportional to its mass, Since the SDS molecules are negatively charged, the result of this is that all of the proteins will have approximately the same mass-to-charge ratio as each other.The gel therefore acts like a molecular sieve when the current is applied, separating the proteins on the basis of their molecular weight with larger proteins being retained higher in the gel and smaller proteins being able to pass through the sieve and reach lower regions of the gel.
https://www.youtube.com/watch?v=JqFmnxGMc2g

No comments:
Post a Comment