Proteins fold up into specific shapes according to the sequence of amino acids in the polymer, and the protein function is directly related to the resulting 3D structure.

The mode of action of chymotrypsin explains as hydrolysis takes place in two steps.
The structure of chymotrypsin selectively cleaves aromatic amino acids due to the hydrophobic pocket at the active site. This means that only sections of the protein that are hydrophobic (such as aromatic portions) will favorably go into this pocket for the reaction to occur.
Proteins may also interact with each other or other macromolecules in the body to create complex assemblies. We will understand the relationship between structure and function in proteins with the help of chymotrypsin (a proteolytic enzyme) and haemoglobin (oxygen carrying protein)
CHYMOTRYPSIN (a proteolytic enzyme):
Chymotrypsin is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Specifically chymotrypsin cleaves phenylalanine, tyrosine, and tryptophan bonds, or in other words the aromatic amino acids. It cleaves these amino acids starting from the C-terminus of the protein.
But this chymotrypsin does not cleave cellular proteins within the pancreas itself because it is synthesized as inactive harmful precursors called chymotrpsinogen (a zymogen).
Chymotrypsinogen must be inactive until it gets to the digestive tract. This prevents damage to the pancreas or any other organs. It is activated into its active form by another enzyme called trypsin. This active form is used to create α-chymotrypsin
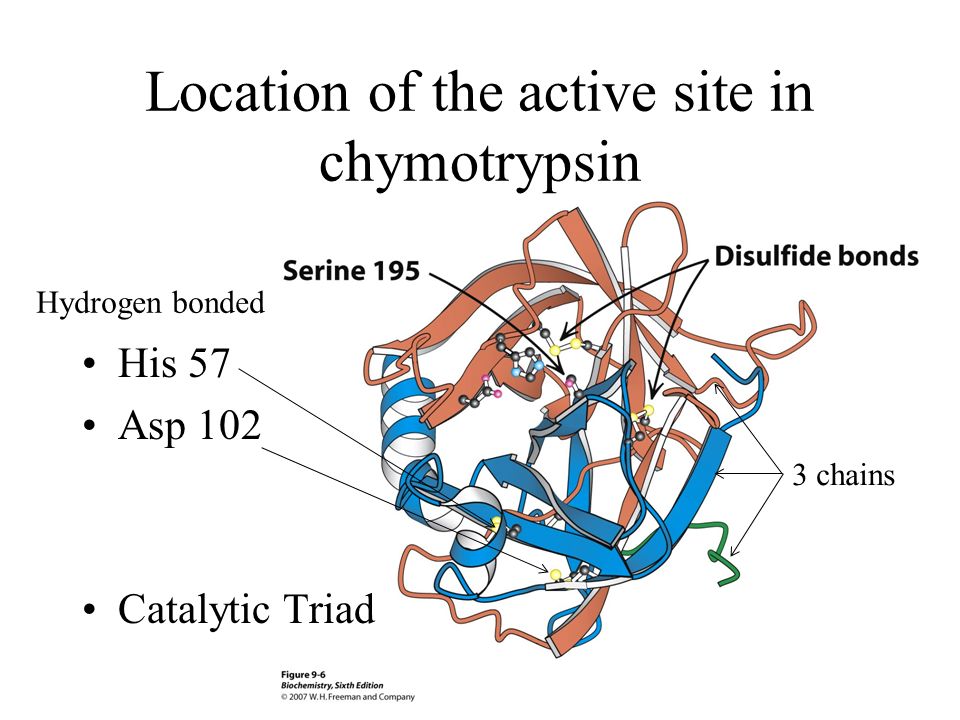
Chymotrypsinogen, is single polypeptide chain of 245 amino acids residues, is converted to alpha-chymotrypsin, which has three polypeptide chains A,B and C linked by two of the five disulfide bond present in the primary structure of chymotrypsinogen

In the active site there are three amino acids which participate in the reaction: aspartic acid, histidine, and serine forms a catalytic triad.
A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes

The mode of action of chymotrypsin explains as hydrolysis takes place in two steps.
The structure of chymotrypsin selectively cleaves aromatic amino acids due to the hydrophobic pocket at the active site. This means that only sections of the protein that are hydrophobic (such as aromatic portions) will favorably go into this pocket for the reaction to occur.
Step 1
First the oxygen on the aspartic acid draws electrons away from the histidine by sharing the hydrogen with the nitrogen. This causes the other nitrogen in the ring to take the hydrogen away from the OH on serine. The negative charge on this oxygen can then attack the peptide bond, with the extra electrons going onto the oxygen
Step 2
When the electrons from the oxygen's negative charge reform the carbon-oxygen double bond the carbon-nitrogen bond of the peptide is broken. The nitrogen takes the hydrogen from the nitrogen on histidine. This releases this portion of the protein, it has now broken off from the rest of the protein:

No comments:
Post a Comment